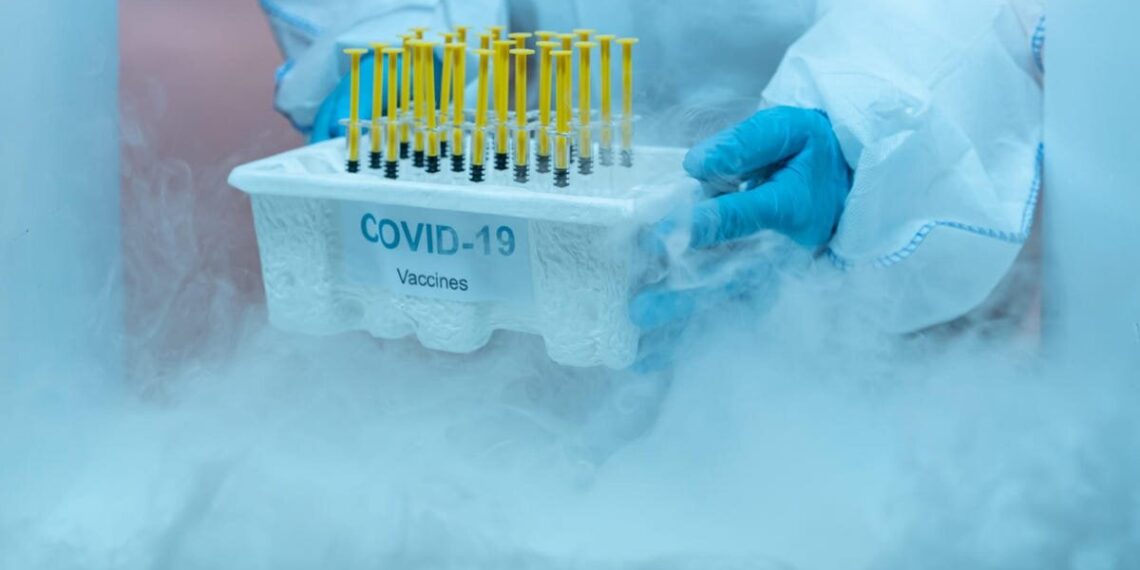
The Nigerian Government on August 24, announced that it had excluded eight of the 36 states in the country from receiving the Moderna vaccines for the second phase of the COVID-19 Vaccine roll-out, because they lack requisite storage capacity, especially in terms of having a backup cold chain facility.
During the first roll-out of the vaccine, all states across Nigeria received the AstraZeneca vaccine because unlike the Moderna vaccine, it can be stored for up to six hours once removed from the fridge.
The Executive Director/Chief Executive Officer of the National Primary Health Care Development Agency (NPHCDA), Dr Faisal Shuaib, while speaking at the joint briefing on COVID-19 vaccination by his agency, the United Nations Children’s Emergency Fund (UNICEF) and the World Health Organization (WHO), explained that the affected eight states did not demonstrate readiness in terms of their cold chain capacity.
“While on the one hand, they have the ultra cold chain that the Federal Government supplied, but we also require that they have backup cold chain equipment,” he stated.
The decision of the Federal Government affirms the claims by some Nigerians, who have shared their concerns on various social media platforms, about the alleged lack of adequate storage facilities for the various types of COVID-19 vaccines in the country.
Why Vaccines?
Having been identified as the needed medication in the battle against COVID-19, countries across the globe have been making efforts to secure vaccines to keep their citizens safe and ensure activities return to normal.
Vaccine distributions to countries have been made possible through donations by developed countries and initiatives like the COVAX facility, a partnership between CEPI, Gavi, UNICEF and WHO, which seeks to donate vaccines to 20% of Africa’s population by the end of 2021.
As at 12 August 2021, a total of 4,428,168,759 vaccine doses have been administered globally while 4,263,958 doses have been administered across Nigeria with 1,417,537 people fully vaccinated as at August 30, 2021.
Available Vaccines, Efficacy and Storage Requirements
Four of the approved vaccines being received in various countries are Janssen, Moderna, Pfizer and Oxford AstraZeneca.
The Janssen COVID-19 vaccine popularly known as Johnson and Johnson is a single dose use with an efficacy of 66.9% against symptomatic moderate and severe COVID-19 (SARS-CoV-2) infection. It is stored between 2°C and 8°C (36°F and 46°F) and the storage unit temperatures require regular monitoring.
The Moderna vaccine can be stored in a freezer at -25°C to -15°C while unpunctured vials may be stored between 8° to 25°C (46° to 77°F) for up to 24 hours. It can not be refrozen once thawed and it has an efficacy of approximately 94.1 per cent in protecting against COVID-19, starting 14 days after the first dose.
The Pfizer vaccine is a 2-dose series separated by 21 days with an efficacy of 95% against symptomatic COVID-19 (SARS-CoV-2) infection. It can also be refrigerated between 2°C and 8°C. The vaccine needs to be kept at -70°C (-94F) or below. Maximum shelf life is 6 months stored in a freezer at -80°C to -60°C
The Oxford Astrazeneca vaccine has an efficacy of 63.09% against symptomatic COVID-19 (SARS-CoV-2) infection with a 2 dose within 8 to 12 weeks range. Once removed from the fridge, it may be stored between 2 to 25°C for up to 6 hours.
Vaccines Received in Nigeria
On March 2, 2021, 3.94 million doses of the AstraZeneca/Oxford vaccine, manufactured by the Serum Institute of India (SII) were delivered to Nigeria through the COVAX facility. Recipients of the AstraZeneca/Oxford vaccine are required to take two doses given intramuscularly (0.5ml each) with an interval of 8 to 12 weeks.
Also, the United States of America through the U.S. Embassy in Abuja, on August 2, 2021, donated four million doses of the Moderna COVID-19 vaccine to Nigeria, as part of the United States’ global efforts to fight the COVID-19 pandemic.
On the list of vaccines received in Nigeria was the 177,600 doses of Johnson & Johnson vaccines delivered to Nigeria on August 8, 2021. The vaccine also requires a single dose.
As of August 17th, Nigeria confirmed the receipt of 699,760 doses of the AstraZeneca-Oxford COVID-19 vaccine donated by the UK government via the COVAX facility.
As part of its commitment to combat the spread of the deadly virus, the Nigerian government in April, proposed to spend about N296 billion for the purchase of coronavirus vaccines.
Efforts are still ongoing to ensure that more vaccines are received in Nigeria, with the aim of increasing the number of people to be vaccinated.
Does Nigeria Have Adequate Storage Facilities?
There have been concerns from Nigerians and even the government, about the storage facilities of the COVID Vaccines especially as the various vaccines require different temperature levels to keep them safe for use.
The Executive Director of the NPHCDA, Dr. Shuaib had revealed that the Federal Government will not distribute vaccines to states that lack backup storage facilities to keep the Moderna vaccines safe and potent.
He noted that, though the Federal Government had distributed ultra-cold chains to states, it is imperative that all the states have backup cold chain equipment.
Dr shuaib said, “while, on the one hand, they have the ultra-cold chain that the Federal Government supplied, we also require that they have backup cold chain equipment. So, either they have a working cold room or a working freezer or chest freezer.
“If, for example, there is a power outage, there is the opportunity to quickly transfer the vaccines to a backup source. That way, we do not risk the potency of the vaccines.
“For these eight states that have not provided that type of backup plan, we are holding on to the vaccines to ensure that they are adequately prepared before we send the vaccine.
“This is in line with our commitments to make sure that only states that have potent vaccines are administered to all Nigerians. Any state that is not ready, we do not deploy the vaccines.
“We are hoping that in the next couple of days, the states will be able to put their acts together and make sure that they are ready to receive the vaccines.”
Dr. Tuyi Mebawondu, a Public Health Expert affirmed that there are varying storage requirements for the vaccine and specifically noted that the Moderna vaccine cannot stay longer than 6 hours outside a fridge which means it requires proper storage.
Dr. Nebawondu however said that before Nigeria receives any vaccine, the donating facility would have assured that there are adequate facilities to preserve such vaccines.
“Before the vaccine leaves where it is coming from, either the COVAX facility or US or UK government, they would have assured there is adequate storage. The National Primary Health Care Development Agency, NPHCDA on its part would have also ensured that adequate facilities are available at the various vaccination centres,” he explained.
He said that storage facilities and logistics arrangement could be responsible for the suspension of the roll out of vaccines which was earlier slated for August 9 before being moved to August 16.
Reacting to concerns about administering vaccines that have thawed and are no longer safe for use, Dr. Mebawondu explained that although there is a lot of distrust regarding COVID Vaccines, he said no public health official will administer vaccines that have not been certified good or not adequately preserved.
He stressed that “there are measures put in place to avoid administering vaccines that have lost potency, which reiterates that nobody will administer an expired vaccine.”
The public health expert noted that, “the reason people are concerned about the storage facilities is because the government is not giving adequate information to the public.”
Implications of Doctors’ Strike on Vaccine Rollout
The National Association of Resident Doctors on August 2 began an indefinite strike over grievances that include the delayed payment of salaries and allowances.
Responding to whether doctors’ strike would affect the vaccine roll out, Dr. Samson Abanni said, “the distribution of the vaccine falls under the purview of public health physicians who are also on strike.”
“Although many workers in the distribution chain are not doctors, many centers for administering the vaccine are in hospitals and the strike has turned the hospitals to ghost towns.
“This means it will definitely affect vaccine distribution but it won’t stop it,” he added.
Dr Boyo Omadeli, public health consultant, while speaking on ‘This Morning’, a program on TVC News on August 9, 2021 believes that, “the doctors’ strike is not healthy, it is not even good for the fight against the pandemic.
“We cannot talk about the deployment of the COVID-19 vaccines without looking at doctors on strike. It is important that the Federal Government hears them out most importantly because of Nigerians who would suffer for it.”
Keeping COVID-19 Vaccines Safe
The Centre for Diseases Control recommends that every COVID-19 vaccine storage facility should have a temperature monitoring device which gives accurate storage unit temperature information, including details on how long a unit (vaccine) has been operating outside the recommended temperature range.
Dr. Samson Abanni noted that, “beyond distributing vaccines to states with back up storage facilities, measures need to be put in place to ensure that the vaccines are well preserved. It is one thing to have a facility, it is another thing to utilize it appropriately.”
“Are we also saying that, whenever there is power outage, the vaccines should be moved to the backup storage and when power is restored, the vaccines should be moved to the main storage. This is a long process which could also alter the potency of the vaccines.”
Dr. Abanni thereafter advised that, “it is essential that cold chain storage facilities across all states in Nigeria have temperature monitoring devices. With this, the potency of a vaccine would not be determined by mere perception but accuracy.
“This OUTBREAK story was supported by Code for Africa’s WanaData program as part of the Data4COVID19 Africa Challenge hosted by l’Agence française de développement (AFD), Expertise France, and The GovLab“